The Robison Lab studies gene transcription and epigenetic regulation in neuronal and immune cells to better understand etiology and treatment of addiction, depression, allergy, and infection.
Circuit-specific gene expression in reward and sex-specific stress responses
When the Robison Lab began at Michigan State in 2013, our initial focus was to characterize the role of FosB gene expression in the hippocampus in learning and memory and drug reward. We were lucky to receive a Whitehall Foundation Research Grant that supported these early studies, and as a result, we were the first to show that ΔFosB is induced by spatial learning in the dorsal hippocampus and is necessary for many different forms of learning (Eagle et al., J Neurosci, 2015). These initial studies led us to explore the role of ΔFosB in the ventral hippocampus, where we showed that it was induced by exposure to stress and drugs of abuse. In 2016, we received an R01 from NIMH to study ![]() ΔFosB regulation of gene expression in ventral hippocampal projections to nucleus accumbens (vHPC-NAc), as this circuit is critical for both drug seeking and stress responses. In collaboration with Rachel Neve, we developed a CRISPR-based intersecting viral strategy that allowed us to perform the first circuit-specific genomic editing in the living mouse brain.
ΔFosB regulation of gene expression in ventral hippocampal projections to nucleus accumbens (vHPC-NAc), as this circuit is critical for both drug seeking and stress responses. In collaboration with Rachel Neve, we developed a CRISPR-based intersecting viral strategy that allowed us to perform the first circuit-specific genomic editing in the living mouse brain.
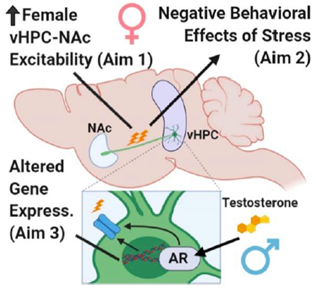
We found that CRISPR knockdown of FosB expression in vHPC-NAc drove susceptibility to social withdrawal following stress while knockdown in other circuits had different behavioral consequences (Eagle et al., Nat Comm, 2020). When we expanded this project to include female mice, we made the startling discovery that the excitability of vHPC-NAc cells is lower in naïve male mice than in females, and that this difference is dependent on testosterone and drives resilience to stress-induced anhedonia (Williams et al., Biol Psych, 2020). These studies resulted in the renewal of the NIMH R01 in 2022, and we are now pursuing the signaling mechanisms and downstream gene targets of androgen receptors in distinct brain circuits driving sex differences, stress responses, and drug seeking.
Gene expression in mast cells in mouse
physiology and behavior
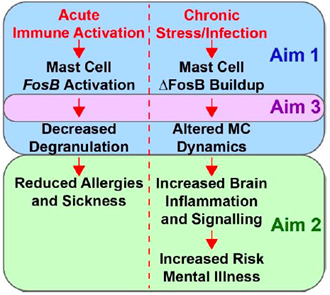
Mast cells are innate immune cells critical for orchestrating responses to infection and immune challenge, and their dysfunction is central to allergies and vulnerability to infection. In 2018, we began a collaboration with Dr. Adam Moeser to uncover mechanisms regulating gene expression in mast cells and the role of mast cells in brain function and behavioral responses to stress. This collaboration was funded by an R01 from NICHD focused on early life stress effects on mast cells, and that funding allowed us to establish that meningeal mast cells regulate immune activity in the meninges and brain (Duque-Wilckens et al., Brain Behav Immun, 2022), and we have now shown that the transcription factor ΔFosB is expressed in mast cells upon activation and serves as a negative feedback mechanism for mast cell activity.
We created mouse lines lacking FosB expression in mast cells, lacking mast cells entirely, and allowing mast cell deletion via diphtheria toxin, and we completed the first measurements of mast cell calcium dynamics in meninges of a living mouse. All of these advances led to a new R01 from NIAID that will allow our groups to pursue this line of research and publish all of these exciting new results in the coming years.
Other Projects
Our research program has become quite broad, and we collaborate with many labs within MSU and across the country. We have a long-standing collaboration with Dr. Gabby Rudenko at UT Medical Branch in Galveston, TX, investigating the regulation of ΔFosB structure and function by oxidative stress and searching for novel compounds inhibiting or activating ΔFosB. This collaborative project is funded by a NIDA R01 and an administrative supplement, allowing us to pursue this work in the context of both drug addiction and Alzheimer’s disease. We collaborate on an R01 led by Dr. Jin He in the MSU Biochemistry department investigating epigenetic control of brain development in autism. Finally, we work with Dr. Brian Trainor at UC Davis on multiple projects. The first is to characterize the physiological properties of oxytocin neurons in multiple brain regions and their role in sexually dimorphic stress responses. The second investigates the role of androgen receptor activity during development in sexually dimorphic adult responses to stress in the California mouse, peromyscus.
